Abstract
Background:R-CHOP is the standard first-line therapy of diffuse large B cell lymphoma (DLBCL). Elderly patients have poor tolerance to chemotherapy, requiring less toxic targeted therapy. Ibrutinib, a first-in-class bruton's tyrosine kinase inhibitor (BTKi), demonstrated considerable efficacy combined with R-CHOP in patients aged < 65 years old with non-germinal center B-cell-like (non-GCB) DLBCL in the PHOENIX study. Lenalidomide, an oral immunomodulatory agent, improved progression-free survival (PFS) in DLBCL patients with higher-risk disease. The Smart Start trial demonstrated the combination of ibrutinib, rituximab, and lenalidomide resulted in impressive response rates in newly diagnosed non-GCB DLBCL. However, ibrutinib was associated with increased unexpected toxicity in elderly patients. Zanubrutinib and orelabrutinib have better target selectivity. To evaluate the safety and efficacy of zanubrutinib/orelabrutinib in combination with rituximab and lenalidomide (BTKiR2), a chemo-free regimen, for elderly patients with newly diagnosed DLBCL, we conducted this single-center prospective study.
Methods: We enrolled patients aged ≥ 65 years old with newly diagnosed DLBCL. Therapy consisted of zanubrutinib 160mg orally twice daily from day 2, or orelabrutinib 150mg orally daily from day 2, rituximab 375 mg/m2 intravenously guttae day 1, and lenalidomide 10-20 mg orally days 2-15 of a 21 day cycle for 6 cycles. The primary endpoint was complete response ratio (CRR) made by PET-CT scan at the end of Cycle 4 and Cycle 6. Patients who had complete response (CR) or partial response (PR) at the end of Cycle 6 received maintenance therapy with lenalidomide 10-20 mg orally days 1-21 in every 28 days for a maximum of 2 years. The secondary outcome measures were overall survival (OS), PFS, and incidence rate of adverse events.
Results: The protocol accrued 38 patients from June 2020 - March 2022, with 37 patients evaluable for disease response. The median age was 75.0 years (range: 65-87), and 37.8% were female. 62.2% had poor risk of R-IPI, 67.6% had advanced stages, and 75.7% had a Ki-67 of ≥ 80%. 24.3% were GCB subtype, and 16.2% of patients were "double expressor". 21.6% (n=8) patients were treated with the OR2 regimen, and 78.4% (n=29) patients received the ZR2 regimen.
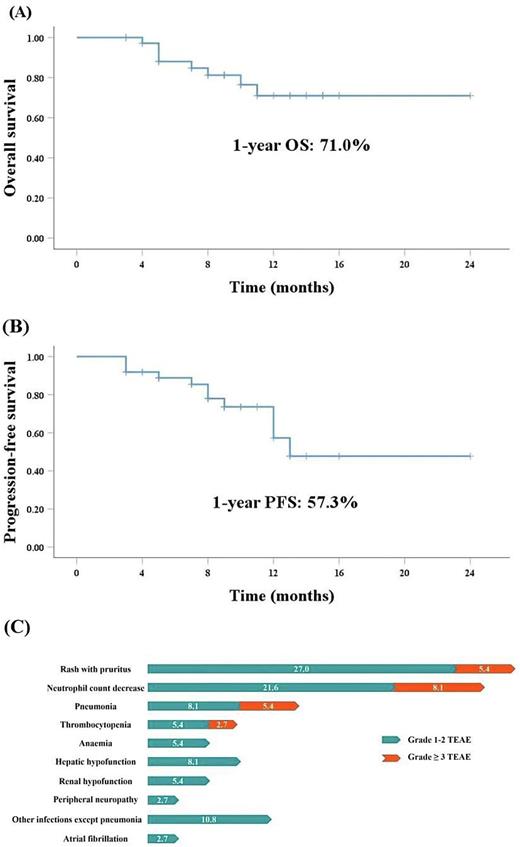
The CRR was 64.9% (n=24). The median follow-up was 9 months (3 - 24m), with 1-year PFS estimate of 57.3% (95% CI 12.8-19.8) and OS estimate of 71.0% (95% CI 16.3-22.1). 24 patients received maintenance therapy and 16.7% (4/24) experienced relapse. The median relapse time was 4.5 months. In the patients (n=15) with high risk of CNS-IPI, 6.7% developed central nervous system lymphoma, and 86.6% avoided lumbar puncture. Patients without B symptoms (P= 0.042) or normal LDH (P= 0.035) achieved higher CRR. The response and survival were not different based upon disease stages, cell of origin, and Ki-67%. All patients (n=3) with the MCD subtype (MYD88 and CD79B double mutations) achieved CR. In patients with TP53 mutations (n=5), one experienced relapse 4 months after the end of Cycle 6, one with SD was shifted to chemotherapy, and the remaining three patients were still in CR at abstract submission. One patient had a CARD11 mutation detected after assessment of PD, indicating NF-κB activation. 42.9% (3/7) gastrointestinal lymphoma patients with SD or PD were shifted to chemotherapy. One patient with cardiac lymphoma (GCB subtype) and two EBV-positive patients had poor response. One patient with a concurrent diagnosis of DLBCL and lung adenocarcinoma achieved CR in lymphoma.
The most frequently reported treatment-emergent adverse events (TEAEs) were rash with pruritus (32.4%) and neutrophil count decrease (29.7%). Grade ≥ 3 TEAEs were neutrophil count decrease (8.1%), rash with pruritus (5.4%), thrombocytopenia (2.7%) and pneumonia (5.4%). The incidence of atrial fibrillation was 2.7% (1/37). No grade ≥ 3 bleeding events were reported. Only one patient discontinued treatment due to grade 3 rash.
Conclusions: TheBTKiR2 regimen demonstrates impressive response rates, survival and safety in elderly patients with newly diagnosed DLBCL compared with conventional chemotherapy, which may also provide protection of CNS against lymphoma infiltration. However, long-term efficacy of BTKiR2 remains unclear. The predictors of poor prognosis are B symptoms and LDH increased. TP53 mutations may be associated with a high risk of relapse.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
BTK inhibitors are approved for MCL and CLL/SLL. Ibrutinib has been demonstrated considerable efficacy in patients aged < 65 years old with non-GCB DLBCL. As a new generation of BTKi, zanubrutinib and orelabrutinib have better target selectivity. We supposed that zanubrutinib and orelabrutinib should be more effective and less toxic in DLBCL than ibrutinib, so we prescribed zanubrutinib and orelabrutinib in this study. Lenalidomide is approved for multiple myeloma. Lenalidomide has been demonstrated considerable PFS in activated B-cell-like subtype of DLBCL patients with higher-risk disease, so we prescribed lenalidomide in this study.
Author notes
Asterisk with author names denotes non-ASH members.